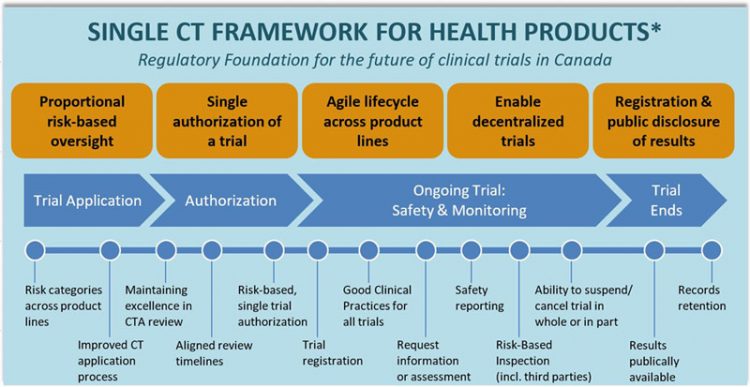
Health Canada Din
Drug identification number DIN linked to the entry for the product in the Drug Product Database which includes the product monograph. Translations of the document are the responsibility of the sponsor involved.
On The Mark Disinfectant Sanitizer Hand Soap Bottle Alcohol Spray Bottle
While Health Canada reviews the product monograph or the veterinary labelling as part of the drug review process it remains the responsibility of the drug sponsor to ensure that the product monograph or the veterinary labelling is complete and accurate.

Health canada din. While Health Canada reviews the product monograph or the veterinary labelling as part of the drug review process it remains the responsibility of the drug sponsor to ensure that the product monograph or the veterinary labelling is complete and accurate. Generic drug manufacturers must update their PM to ensure it aligns with the Canadian Reference Product. Is medical cannabis covered through insurance.
Labels for animal drugs. Here are the elements specific to each DIN. Health Canada provided overviews of the situation relating to nitrosamine impurities in pharmaceuticals and.
This includes pharmaceuticals for human and veterinary use as well as disinfectant. They are intended to assist in preparing drug submissions when seeking an approval to sell a pharmaceutical drug product in Canada. 11 rows All disinfectants that have a drug identification number DIN have been approved for sale.
02410605 Electronic product monograph is not available Company. Product monograph PM for human drugs. Health Canadas drug review and approval process nor does it have a Drug Identification Number DIN or Natural Product Number NPN.
2019-05-13 Original market date. Marketed Current status date. The placement of the DIN on the package label provides evidence that the product has been evaluated and authorized for sale in Canada.
How the drug or vaccine was authorized. The DPD is updated nightly and includes. Availability of the drug in Canada.
A Drug Identification Number DIN is an eight-digit number assigned by Health Canada to a drug product prior to being marketed in Canada. Therapeutic area using the World Health Organizations coding system Health Canadas control number for the application. From Health Canada.
While Health Canada reviews the product monograph or the veterinary labelling as part of the drug review process it remains the responsibility of the drug sponsor to ensure that the product monograph or the veterinary labelling is complete and accurate. 20091229 13 Scope and Application This guidance applies to drugs regulated under Part C Division 1 of the Regulations that have received a DIN pursuant to Section C010142. For industry information about COVID-19 visit our COVID-19 Drugs and vaccines section.
Webinar on Nitrosamines January 31 2020. What role can pharmacists play in medical cannabis. The Product Monograph Brand Safety Updates table.
Marketed Current status date. 2018-10-17 Original market date. A copy can be requested by contacting hcbpsenquiriessccanadaca.
2018-11-06 Original market date. Search the Drug Product Database DPD to find drugs authorized for sale by Health Canada. The purpose of this session was to provide an opportunity for a discussion between Health Canada and stakeholders.
Post-Drug Identification Number DIN Changes Health Canada Guidance Document 2 Date Adopted. Marketed Current status date. Guidance documents have been prepared to assist in the interpretation of policies and governing statutes and regulations.
You may search by either a drug identification number DIN b Anatomical Therapeutic. Some insurance plans may cover medical cannabis. Health Canada has seized and warned Canadians about several unauthorized skin lightening health products whose labels show they contain prescription drugs eg betamethasone dipropionate clobetasol propionate or hydroquinone at concentrations greater than 2.
From Health Canada. 2014-04-14 Original market date. A Drug Identification Number DIN is a computer-generated eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada.
Check each patients individual plan for more details. Even though pharmacists are not dispensing. Marketed Current status date.
Due to the fact that the information originated with an organization that is not subject to the Official Languages Act the document may only appear in the language in which it was written. The letter was sent to all DIN and DEL holders.
Vintage Bayer Aspirin Tin Can Mini Pill Box 80s Made In Tin Can Aspirin Pill
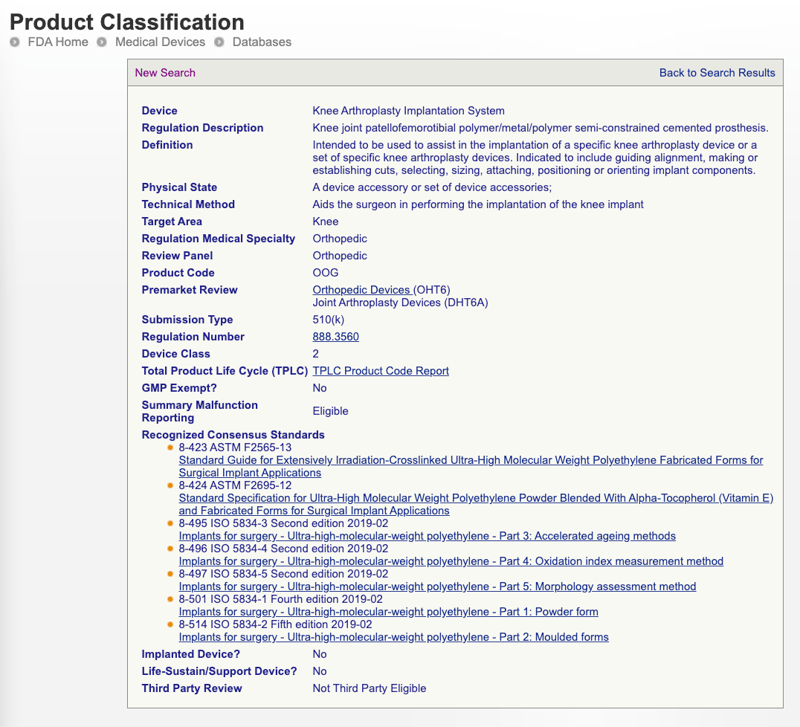
You Can Identify Licensed Natural Health Products By Looking For The Eight Digit Natural Product Number Npn O Homeopathic Medicine Natural Health Homeopathic
Flor Essence Flora Health Canada En Whole Body Cleanse Herbal Cleanse Flor Essence
Amazon Com Nasol Spray 30ml Replaces Sinus Buster Brand Bionas Sinus Rinse Treatments Health Personal Care Sinusitis Sinus Allergies Sinus Rinse
Advisory Allerject Auto Injector Recall Allergies Food Allergies Epinephrine
Thyroid Erfa Canada 2012 Inc Thyroid Thyroid Hypothyroidism Thyroid Tablets
Fysiurgisk Massor Tag Hand Om Din Krop Medical Anatomy Brain Anatomy Medical Knowledge
Pin On Skin Care Let Beauty Canada
Downhill Ski Bindings Din Chart Powder7 Ski Bindings Skiing Skis For Sale
Saniblend 32 Is One Of Our Go To Products Here At Simcoe Green Go To Our Website To Learn More About It Biodegradable Products Green Solutions Cleaning Hacks
Health Canada Recalls Allerject Auto Injector Products Cbc News Allergies Epinephrine Food Allergies
Canadian Healthcare Network Health Care Allergic Rhinitis Pharmacist


Posting Komentar untuk "Health Canada Din"