Health Canada Medical Device License Search
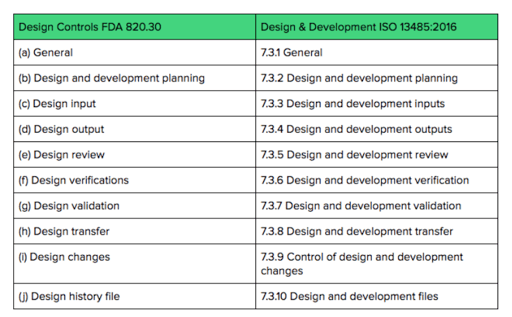
Medical Devices Licence Listings. Health Canada originally mandated that these companies would be required to register their companies to the ISO 13485 standard as the software is a medical device.
Complete Guide To Bringing A Medical Device To Market
Canada Medical Device Regulations.
Health canada medical device license search. Also search for a licensed device using the listing database. Promotes care team collaboration and drives better patient outcomes. Device Licenses for Ultrasonic Diagnostic Systems and Transducers Health Canada the countrys regulating authority in the sphere of medical devices and other healthcare products has published a guidance document dedicated to medical device license applications for ultrasound diagnostic systems and transducers.
Health Canada is the Federal department responsible for reviewing medical devices and in vitro diagnostic devices IVD devices to assess their safety effectiveness and quality before being authorised for sale in Canada. An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet the safety requirements set out. A medical device that is manufactured.
Medical Devices Medical Information Technology Medical Software and Health Informatics. Also search for a licensed device using the listing database. Health Canadas guidance on Medical Device Compliance and Enforcement GUI-0073 states that if a regulated party does not voluntarily respond to Health Canada requests such as inspections request for copies of procedures etc to comply with the Medical Devices Regulations measures can be considered including the suspension of an establishment licence.
Please select all that apply. Obtaining an MDL is comparable to the US FDA 510 k process. Draft Health Canada IMDRF table of contents for medical device applications guidance 2019-02-28 Implementation of Advance Notice of Importation Process for Medical Devices 2019-02-12 Guidance on Advance Notice of Importation under section 211 of the Medical Devices Regulations MDR and section 31 of the Radiation Emitting Devices Regulations REDR 2019-02-12.
Health canada medical device license. Health Canada guidance documents policies reports directives applications forms fee and export materials related to establishment licences for drugs medical devices pharmaceutical products and natural health products. Selecting the Active Licence Search link takes you to the Medical Devices Active Licence Search window.
Hello all Just an FYI for all those companies manufacturing electronic medical records software. If you choose to sell through distributors in Canada. Medical Devices Active Licence Listing MDALL Purchase of Licensed Medical Devices for Use in Health Care.
A Medical Device Establishment Licence MDEL is a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada. 412020 Medical Device Licence MDL a licence issued to manufacturers authorizing them to import or sell their Class II III or IV medical devices in Canada. Medical Device Related Regulations.
There are two types of licenses issued by Health Canada with different requirements. 472008 Health Canada Medical Device License MDL A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada. The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada.
HC has defined these medical devices as a. Medical Devices Active Licence Listing MDALL Health 5 days ago Medical Devices Active Licence Listing MDALL - Your reference tool for licensed medical devices in CanadaFrom Health CanadaDear visitor We have reorganized our Web site. Medical devices and in vitro diagnostic devices IVDs are regulated By Health Canada HC under the Canadian Medical Devices Regulations.
Health Canada Medical Device Establishment License MDEL If you manufacture Class I medical devices or In Vitro diagnostic devices IVDs and plan to sell directly into Canada without a distributor you must secure a Medical Device Establishment License MDEL. A link button or video is. Within Health Canada the Health Products and Food Branchs mandate is to take an integrated approach to minimising the health risk factors to Canadians while maximising.
Its now looking like. In response to the COVID-19 outbreak HC has outlined an Interim Order IO for the importation and sale of medical devices for use in relation to COVID-19. The Medical Devices Bureau Bureau of the Therapeutic Products Directorate Health Canada is the Canadian federal regulator responsible for licensing medical devices in accordance with the Food and Drugs Act and Regulations and the Medical Devices Regulations.
Medical device licensing Access forms and guidance documents to help you apply for a medical device licence. Report a problem or mistake on this page.
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Source Verification Medical Council Of Canada
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Cpr Cpr Training Basic Life Support
Checklist Kahwin Excel 2019 Google Search Checklist Bar Chart Chart
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Get Nurse Email List Registered Nurse Database From Medicoreach Nurse Registered Nurse Email List
Usa Work Permit Documents Yahoo Search Results Image Search Results Canada Tourist British Visa Application Form
Free Job Posting Sites In Canada 25 Best Websites For Canadian Jobs Ads2020 Marketing Job Posting Job Posting Sites Free Job Posting
Pin On Buy Real And Fake Passport Visa Driving License Id Cards Marriage
Complete Guide To Bringing A Medical Device To Market
Canada Passport Psd Template Doctors Note Template Passport Template Psd Templates
Masters Dissertation Questionnaire In 2021 Dissertation Problem Solving Worksheet Essay Outline
Le Manoosh Search Results For Medical Medical Device Design Medical Design Intuitive Surgical
Aplio 500 Platinum Google Search Medical Design Medical Portfolio Design
Source Verification Medical Council Of Canada
How To Search Fda Registration Number Fdabasics
Pin On International Mailing List


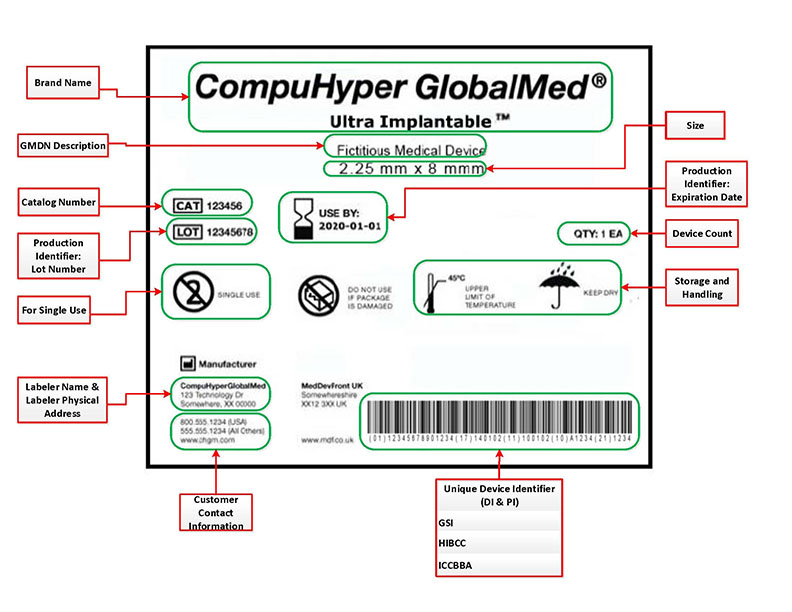

Posting Komentar untuk "Health Canada Medical Device License Search"