Health Canada Class Ii Medical Device Application
Fees for examination of application for an establishment license Medical Devices Medical Devices Establishment License Application for new license and annual review of license 4590 120 calendar days to issue decision Fees for the Right-To-Sell RTS licensed class II III or IV devices Right-To-Sell medical device The annual fee to right to sell medical devices class II III. Obtaining an MDL is.
Toronto Nov 16 2017 Groundbreaking Medical Device Now Licensed With Health Canada Seqex Has Been Approved As A Class Ii Medi Medical Health Medical Device
Health Canadas performance targets were set at 15 days for first decisionsthat is decisions whether to issue MDLs or request additional informationfor Class II Medical Device License MDL applications 60 days for Class III MDL applications and 75 days for Class IV MDL applications.
Health canada class ii medical device application. PurposeIntended Use of Device. Class II III IV Health Canada MDL applications. Health Canada Medical Device License MDL A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada.
Cannot advertise or represent by label a treatment for a Schedule A disease or disorder Section 3 Cannot sell or advertise a device that may cause harm Cannot sell or advertise a device in a misleading or deceptive way All medical devices those used on human beings must also comply. New MDLs mostly on target. Class II - Amendment Licence Application.
Clinical trials for medical devices are not classified by phased development as with drugs ie Phase I-IV clinical. Medical Devices - Service Standards Average Time in Calendar Days. You should visit the Health Canada Web site to get all the latest information related to Canada.
Ad CPD Accredited online Health and Social Care Courses Tutor Support Enrol Now. Draft New Class II Medical Device Licence Application Form - Health Canada httpwwwhc-scgccadhp-mpsconsultationmd-imlicapp_demhom_cla2_draft_ebauche-engphp10222014 104911 AM Name Title. The review of Medical Device Licence Applications new licence the requests for the reinstatement of an existing licence as well as the amendment of a current medical device licence are subject to fees.
Al is correct Class II devices are not exempt. Provide a description of the medical devices covered by this application and their intended use. In addition you will find out that Class 1 devices are exempt that is if you are strictly a.
25 years market expertise. Place of Use Is this device sold for home use. The applicable fees for the fiscal year 2019-2020 expressed in Canadian dollars are reported in the table.
Class II - Licence Application. In Canada Sponsors or Manufacturers should submit an Investigational Testing Application ITA to use unapproved Class II III and IV devices in clinical trials to the Device Evaluation Division of the Medical Devices office of Health Canada. Class II III IV-Investigational Testing Authorization Applications.
Application for a New Medical Device Licence for a Private Label Medical Device 2020-04-01 Bed-related Entrapment and Fall Report Form 2008-03-17 Class II Medical Device Licence Amendment Application Form. In order to market their devices in Canada manufacturers must obtain a license. The application form has a new section requiring information about the phthalate content of the device in the application.
Name of the Device as it appears on the label 2. Fortunately this is a Class 2 device and the requirements are primarily to complete the application form for a new Class 2 device license httpbitlyCanadian-Device-License-Form sign attestations regarding compliance with the safety and effectiveness requirements Section 10-20 of the CMDR and compliance with the labeling requirements Section 21-23 of the CMDR. Class IV MDL applications are comparable to a US PMA application.
Draft Class II Medical Device Licence Amendment Application Form httpwwwhc-scgccadhp-mpsconsultationmd-immd_licam_im_demmhom_cla2_draft_ebauche-engphp10222014 105029 AM Name Title. Regulatory Provisions All devices offered for sale in Canada must comply with the Food and Drugs Act. This guidance provides information to manufacturers and regulatory correspondents on how to complete an application form for a new medical device licence.
Investigational Testing in Human Clinical Trials. To apply for an Authorisation for Investigational Testing of Class II III or IV medical devices on human subjects in Canada a manufacturer andor device sponsor must use the requisite application form 9. Class II III IV-Private Label Applications.
Class II III and IV medical devices must be licenced before they may be imported or sold in Canada. 17 rows For additional information on the legal requirements to support medical device licence. Compared to a US 510 k application MDL applications are simpler for Class II devices and about the same for Class III devices.
Health Canada has four classes not three as we do here and in the EU. We manage the entire application process for Health Canada Medical Device License MDL for Class II III or IV medical or surgical devices IVD POCT-NPT SaMD. For Class II III and IV devices apply for a Canadian Medical Device License MDL application for your device.
Class III - Licence Application. A licence is issued to the device manufacturer for each application submitted provided the requirements of the Medical Devices Regulations are met. The MDL is a product approval while a MDEL is a permit for the companydistributorimporter itself.
There is no need to make an application to Health Canada to conduct investigational testing of Class I medical devices. New Class II Medical Device Licence Application Form disponible en français Before completing this form you must consult the document Guidance Document How to Complete the Application for a New Medical Device Licence available on the website. PDF fillablesaveable 247 K 2020-04-15 Doc Version - 249 K Class IV Medical Device Licence Amendment Application Form.
The supporting documents listed in Section 81 of the Regulations paragraphs ak should accompany the application. Complete items 8 and 9 only if they have changed from the previous licence 8.
Pin By Phillip Fagg On Massage Spa Kinesiology Taping Kinesiology Lymph Vessels
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Drug And Medical Device Highlights 2018 Helping You Maintain And Improve Your Health Canada Ca
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Https Www Who Int Medical Devices Countries Regulations Can Pdf Ua 1
What Is A Medical Device Quality Management System Qms
Cbc Takes You Inside Health Canada S Medical Device Records Medical Medical Device Healthcare Provider
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
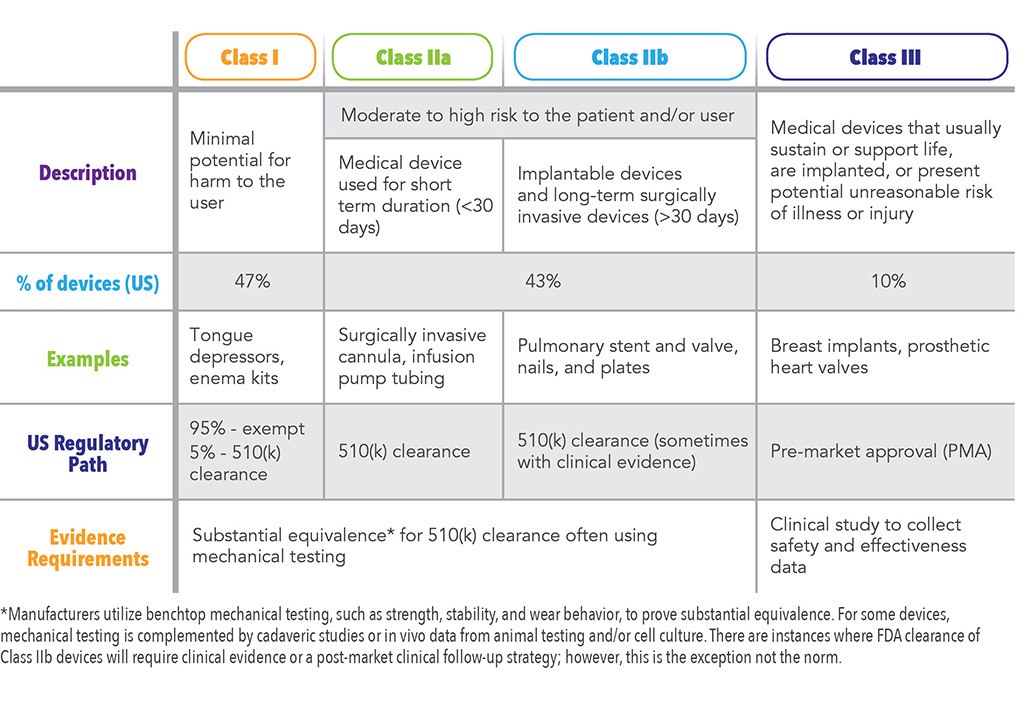
Guidance Document Guidance For The Risk Based Classification System For In Vitro Diagnostic Devices Ivdds Canada Ca
Software As Medical Device Samd Classification And Definitions
Software As Medical Device Samd Classification And Definitions
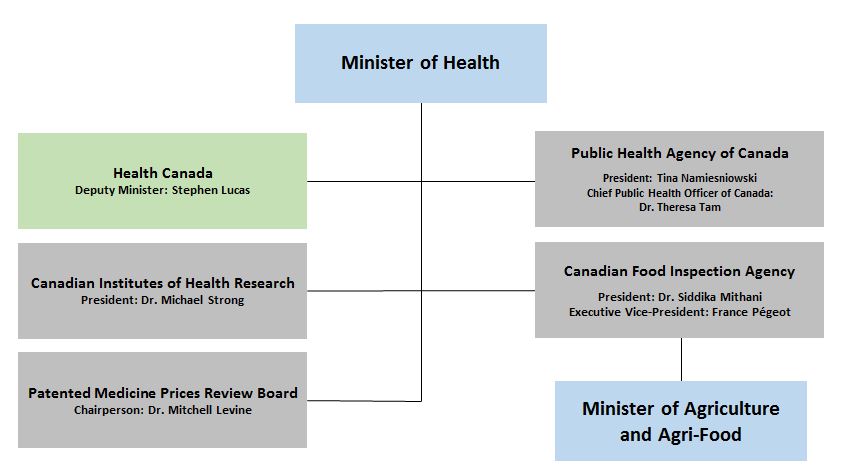
Deputy Minister Briefing Material September 2019 Canada Ca
Cleaning Validation Guidelines A Complete List 2021
Software As Medical Device Samd Classification And Definitions


Posting Komentar untuk "Health Canada Class Ii Medical Device Application"