Health Canada Device Approval
Our Canadian medical device approval process chart offers a detailed look at the steps required for Health Canada approval. 2 states that no person can import or sell a COVID-19 medical device if there are significant differences in the device from that which was initially submitted to Health Canada for approval under either Interim Order No.
Five Trends To Watch In The Medical Device Industry Mercer Capital
Issuing an authorization Health Canada is committed to reviewing clinical trial applications and clinical trial application amendments for COVID-19 medical devices within 14 days.

Health canada device approval. 1 or Interim Order No. Once the application is approved it will be posted on the Health Canada Website. We approved 138 new generic drugs and 3.
There are two types of licenses issued by Health Canada. Drug and health product review and approval - Canadaca Drug and health product review and approval Information on the approval process for drugs medical devices natural health products and homeopathic medicine. The License does not expire.
In Canada medical devices are. Once approved the manufacturer can sell your device in Canada. Medical Devices and IVD Devices are subject to registration.
Obtaining an MDL is. In late 2018 a series of news articles took aim at Canadas regulation of medical devices alleging that Health Canadas medical device approval and adverse event monitoring systems were severely lacking. Once approved the manufacturer can sell your device in Canada.
Health Canada CMDR Quality System Requirements and ISO 13485 Health Canada requires manufacturers of Class II - IV medical devices to meet the QMS of ISO 13485 under MDSAP which includes compliance with the requirements of the CMDR. Health Canada regulators today approved the ID NOW rapid COVID-19 testing device for use in this country a move that could result in millions. Health Canada Medical Device License MDL A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada.
The 1 Medical Device Establishment Licence MDEL required for Class I medical devices and the 2 Medical Device Licence MDL for all the other classes. We are unable to prioritize requests for status updates at this time. The MDL is a product approval while a MDEL is a permit for the companydistributorimporter itself.
The Canadian approval process for IVDs explained To obtain access to the Canadian market in vitro diagnostic device manufacturers will need to secure a license. Once approved the manufacturer can sell your device in Canada. Health Canada issues two types of licenses.
The evaluation of medical device submissions is a service with a high volume of regulatory transactions used by industry and health care professionals for all medical devices imported or sold in Canada. The Medical Devices Bureau Bureau of the Therapeutic Products Directorate Health Canada is the Canadian federal regulator responsible for licensing medical devices in accordance with the Food and Drugs Act and Regulations and the Medical Devices Regulations. For a drug a biologic or a genetic therapy a medical device a combination product a natural health product or other health product company seeking approval of their product for sale in Canada it is important to understand that the approval process is subject to.
Section 6 of Interim Order No. The License does not expire. The Medical Devices Regulations set out the requirements governing the sale importation and advertisement of medical devices in Canada.
To market their devices in Canada manufacturers must obtain a license. Additional information and material including samples that we request is encouraged to be. If you learned recently that a device was authorized please allow at least 48 hours for this list to be updated.
2 unless the Minister has issued an amended authorization. However if the annual fee is not paid the license will be revoked. Includes drug pricing and drug approval decisions.
Health Canada is receiving a very high volume of requests for authorization. This article will provide an update regarding steps Health Canada has now taken to tighten up regulation of medical devices. When a new medical device is approved it is issued a medical device licence.
In 2017 Health Canada approved 67 new drugs including 36 new active substances. However if the annual fee is not paid the license will be revoked. This does not mean the drug or medical device will immediately be available to patients as many other factors can influence that timeline.
The Health Canada Medical Device Establishment License MDEL and the Health Canada Medical Device License MDL.
Technical Documentation Technical File Precondition For Approvals
Best Tips Iso 13485 Procedures With Our Free Template Version 2016 Iso 13485 Iso Procedure Template
Five Trends To Watch In The Medical Device Industry Mercer Capital
Five Trends To Watch In The Medical Device Industry Mercer Capital
Five Trends To Watch In The Medical Device Industry Mercer Capital
Novo Nordisks New Prefilled Insulin Pen Receives Health Canada Approval Pen Design Insulin Medical
Does An Fda Class 1 Medical Device List Exist
Medical Devices Market Regional Analysis 2020 2025 Global Market Size Research Report Trends Growth Medgadget
Five Trends To Watch In The Medical Device Industry Mercer Capital
What Is Samd Everything About Software As A Medical Device
Prrc Or Person Responsible For Regulatory Compliance Mdr 2017 745 Medical Humor Regulatory Compliance Regulatory Affairs
Ypsopump Receives Health Canada Approval Insulin Pump Diabetes Diabetes Care
What Is A Medical Device Quality Management System Qms
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Technical Documentation Technical File Precondition For Approvals
Clinical Trials Medical Device Trials Genesis Research Services
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Why The Rep Less Sales Model For Medical Devices Won T Stick Medical Device Sales Medical Sales Medical


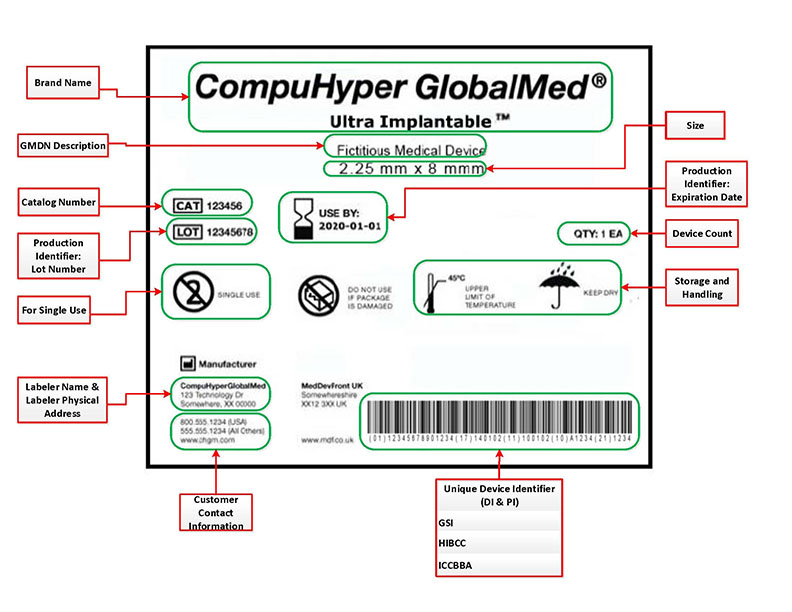
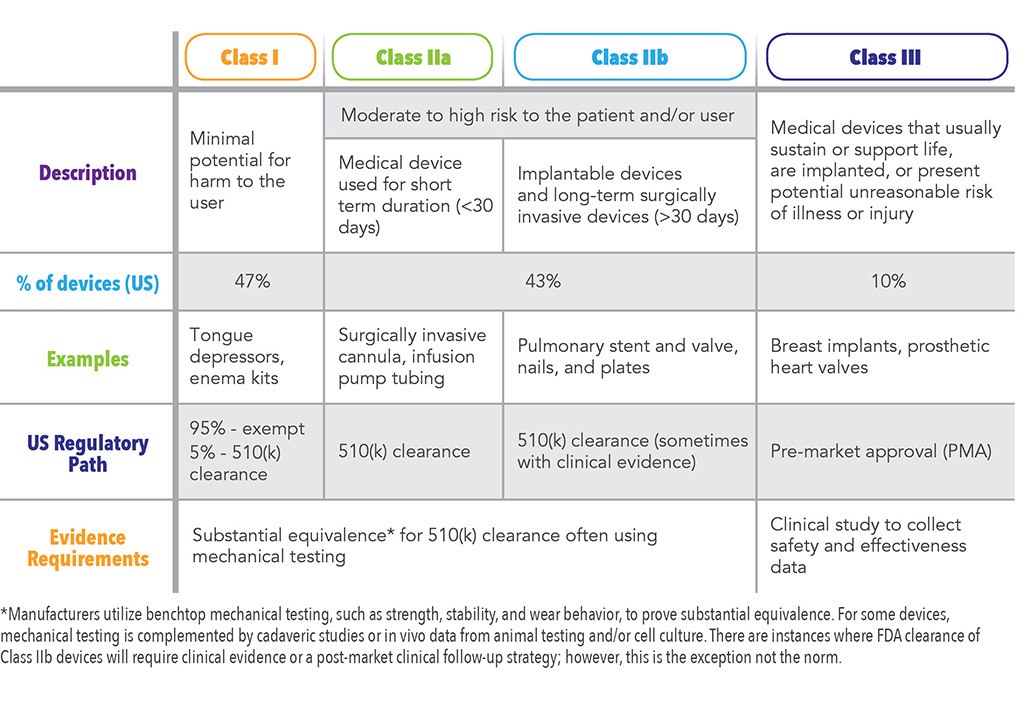
Posting Komentar untuk "Health Canada Device Approval"