Sona Nanotech Health Canada
Sona Nanotechs gold nanorod particles are CTAB cetyltrimethylammonium free eliminating the toxicity risks associated with the use of other gold. SNANF the Company a developer of rapid point-of-care diagnostic tests is pleased to announce that its rapid detection COVID-19 antigen tests laboratory validation studies of performance levels have resulted in a test sensitivity of 96 test specificity of 96 and a Limit of Detection LOD of 2.
Sona Nanotech Health Canada Evaluation Results Did Not Match Prior Analytical And Clinical Study Data The Deep Dive
Sona Nanotech is a nanotechnology life sciences firm that has developed multiple proprietary methods for the manufacture of various types of.
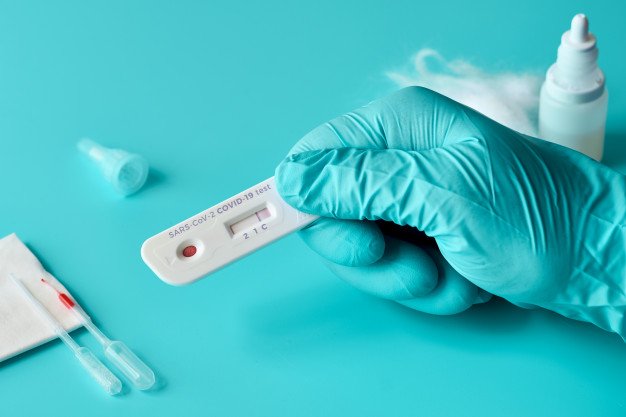
Sona nanotech health canada. A developer of rapid point-of-care diagnostic tests received notice from the FDA that the Companys request for an emergency use. SONA and GE Healthcare Life Sciences will jointly complete test development of the Sona Covid-19 Coronavirus rapid response lateral flow test and will use GE Healthcare Life Sciences Fast Flow High Performance Membrane FFHP in production of the test. Sona_nanotech has received further feedback from Health Canada subsequent to its withdrawal of its application for an Interim Order authorization IO for the marketing of its rapid COVID-19 antigen test.
As part of its review Health Canada commissioned an evaluation from the National Microbiology Laboratory NML whose evaluation produced discordant results to the Companys prior. In what is a major blow to shareholders of Sona Nanotech CSE. It is expected that Sona Nanotechs gold nanotechnologies may be adapted for use in applications as a safe and effective delivery system for multiple medical treatments subject to the approval of various regulatory boards including Health Canada and the FDA.
Shares in Sona Nanotech SONA are plummeting after the company withdrew its rapid COVID-19 antigen test from Health Canadas approval process Based on feedback from the regulatory body Sona has withdrawn the application in order to obtain. The latest tweets from SONANanoTech. Sona Nanotech Inc.
July 2 2020 Halifax Canada Sona Nanotech Inc. About Sona Nanotech Inc. Sona Nanotechs gold nanorod particles are CTAB cetyltrimethylammonium free eliminating the toxicity risks associated with the use of other gold.
SONA the company this afternoon announced that it has withdrawn its rapid COVID-19 antigen application from Health Canada based on feedback it has received from the regulatory agency. The move is being conducted in order to obtain more clinical data to augment its submission. Is a nanotechnology life sciences firm that has developed multiple proprietary methods for the manufacture of various types of gold nanoparticles and used this technology to develop a COVID-19 rapid antigen test.
Halifax Nova Scotia March 3 2020 Sona Nanotech Inc. Sona Nanotechs gold nanorod particles are CTAB cetyltrimethylammonium free eliminating the toxicity risks associated with the use of other gold. Bloomberg the Company Its Products The Company its Products Bloomberg Terminal Demo Request Bloomberg Anywhere Remote Login Bloomberg Anywhere Login Bloomberg Customer Support Customer Support.
Sona Nanotechs gold nanorod particles are CTAB cetyltrimethylammonium free eliminating the toxicity risks associated with the use of other gold.
Sona Nanotech Inc Home Facebook
Welsh Tracking Technology Powers A Covid 19 Rapid Test Bond Digital Health
Nanomaterials Free Full Text Can Nanotechnology And Materials Science Help The Fight Against Sars Cov 2 Html
Sona Partners With Ge Healthcare Life Sciences To Complete Coronavirus Rapid Screening Test Sona Nanotech Sona Nanotech
Sona Nanotech Announces Validation Results For Its Covid 19 Antigen Test
Covid 19 Diagnostic Tests Production Gaps Bioprocess Internationalbioprocess International
Ten Canadian Companies With Rapid Covid Tests Awaiting Health Canada Approval
Covid 19 Testing Authorizations In The Us And Canada Starfish Medical
Sona Nanotech Receives Deprioritization From Fda Health Canada Evaluation Continues Sona Nanotech Sona Nanotech
Covid 19 Diagnostic Tests Production Gaps Bioprocess Internationalbioprocess International
Sona Nanotech Rapid Covid 19 Antigen Test Ce Marked
Sona Nanotech Updates On Timing Of Clinical In Field Validation Studies For Its Covid 19 Antigen Test Statnano
Bond Raises 700 000 To Develop Connectivity For Rapid Covid 19 Tests Bond Digital Health
Sona Nanotech Withdraws Rapid Covid 19 Antigen Test Application Based On Feedback From Health Canada
Covid 19 Test Sona Nanotech Sona Nanotech
Canada Signs Deal To Buy 7 9m Rapid Coronavirus Tests Pending Approval National Globalnews Ca
Covid 19 Global Diagnostic Consortium To Fight Pandemic Bond Digital Health
Snanf Investor Alert Bronstein Gewirtz Grossman Llc Notifies Sona Nanotech Inc Shareholders Of Class Action And Lead Plaintiff Deadline February 16 2021 Business Wire




Posting Komentar untuk "Sona Nanotech Health Canada"