Health Canada Medical Device Establishment License Database
412020 Medical Device Licence MDL a licence issued to manufacturers authorizing them to import or sell their Class II III or IV medical devices in Canada. Class II III and IV devices shall apply for a Canadian Medical Device License MDL application.
5 Tips For Medical Device Registration Across Global Markets
The document requirements for each of the device class vary.
Health canada medical device establishment license database. The Medical Devices Bureau Bureau of the Therapeutic Products Directorate Health Canada is the Canadian federal regulator responsible for licensing medical devices in accordance with the Food and Drugs Act and Regulations and the Medical Devices Regulations. This window is identical to the original MDALL search and displays the results as before. Establishment Licensing EL fees Health Canada inspects establishments to assess whether they comply with regulatory requirements to conduct regulated activities related to drugs and medical devices.
Third the regulator has launched initiatives to boost cost recovery and operating revenues. 20 Legislated Requirements Any person who imports into Canada or sells in Canada a medical device for human use requires an establishment licence with the exception of a retailer a healthcare facility a manufacturer of Class II III or IV medical devices that only. All Canadian drug establishments must hold since January 1 1998 an establishment licence to fabricate package label distribute import wholesale or test a drug.
The MDL is a product approval while a MDEL is a permit for the companydistributorimporter itself. Medical Device Establishment License MDEL. Class I medical devices do not require a medical device licence and are monitored by the Health Products and Food Branch Inspectorate Compliance and Enforcement through Establishment Licensing.
Report a problem or mistake on this page. Health canada medical device license. Following is an excerpt from the Health Canada website.
Medical Devices Medical Device Establishment Licences Medical Devices Establishment Licence Listing MDEL Guidance on Medical Device Compliance and EnforcementGUI-0073. Only products which appear in this database listing may be offered for general marketing purposes in Canada. Class I devices can apply for Medical Device Establishment License MDEL by preparing mandatory procedures and paying Health Canada fees.
Medical Device Establishment Licences. Application for a Manufacturers Certificate to Cover Export of Medical Devices GUI-0097 Guidance on Medical Device Compliance and Enforcement GUI-0073 2015-06-12 Medical Devices Establishment Licence Live Listing. A Medical Device Establishment Licence MDEL is a licence issued to Class I.
Management of Applications and Performance for Drug Establishment Licences GUI-0127 Drug Establishment Licence Application Forms and Instructions FRM-0033. The Licence Number query was improved to return the exact number match only. To view active MDEL holders see the Medical Devices Establishment Licence Listing.
Health Canada Medical Device License MDL A Canadian Medical Device License MDL is required for companies selling Class II - IV medical devices in Canada. MDEL is not required. Medical Device Establishment Licence MDEL a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada.
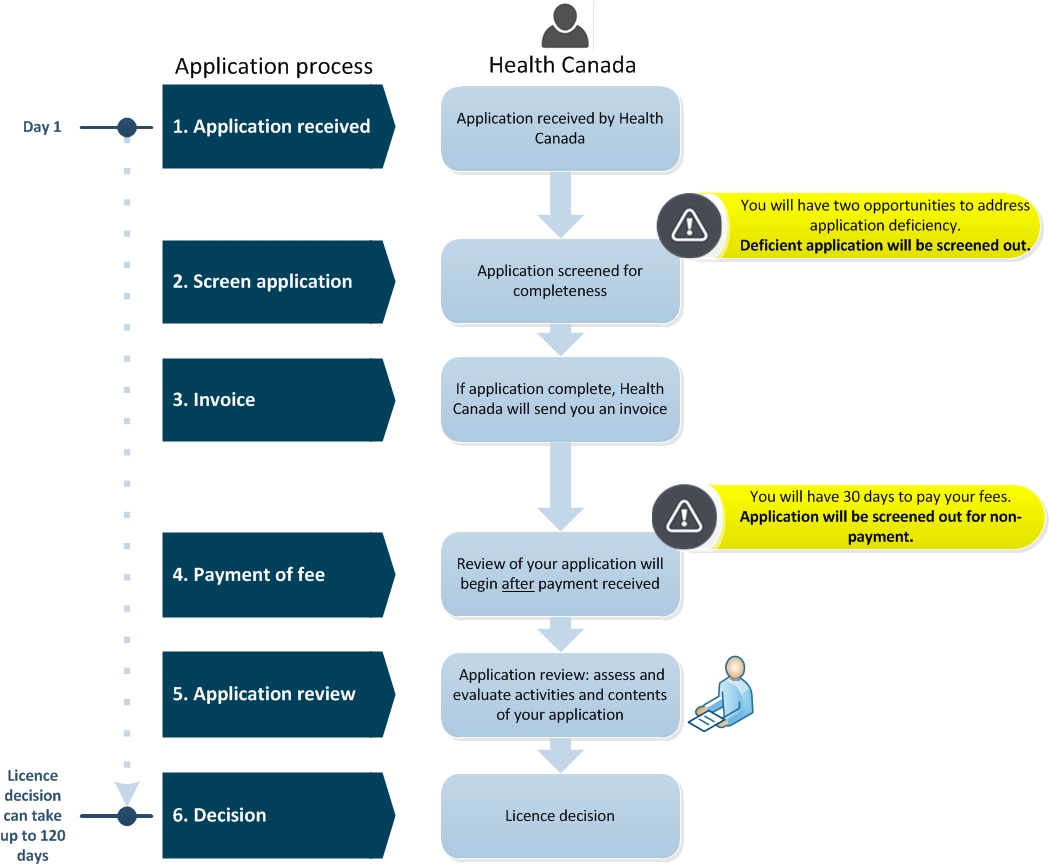
For medical device licenses tracking of these standards begins on the date of acceptance of a license application and lasts for 120 days. From Health Canada. Second Health Canada has established performance standards for issuance of establishment licenses.
Before a drug or medical device is authorized for sale in Canada Health Canada reviews it to assess its safety efficacy and quality. The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada. In particular the authority describes the way the interested party shall apply for a medical device establishment license.
Medical Devices Annual Review Documents. Also search for a licensed device using the listing database. Obtaining an MDL is comparable to the US FDA 510 k process.
A concept based on the Canadian healthcare system. Health Canada the Canadian authority responsible for the regulatory framework for medical devices and other healthcare products has published guidance dedicated to the licenses an entity shall obtain to be allowed to perform certain regulated activities. Medical Device Establishment Licensing information httpwwwhc-scgccadhp-mpscompli-conformlicencesindex-engphp The above link will direct you to these headings.
Dear visitor We have reorganized our Web site. Medical Device License MDL. Selecting the Active Licence Search link takes you to the Medical Devices Active Licence Search window.
The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada.
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Https Www Ihe Ca Download Medical Device And Diagnostic Pricing And Reimbursement In Canada Pdf
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Overview Of The Regulatory System For Medical Devices In Kenya
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Health Canada Introduces New Reporting Requirements To Reduce Shortages 2021 04 14 Fdanews
Https Www Ihe Ca Download Medical Device And Diagnostic Pricing And Reimbursement In Canada Pdf
5 Tips For Medical Device Registration Across Global Markets
Revised Fee Proposal For Drugs And Medical Devices Canada Ca
Private Labeled Devices With Fda Approval Medical Device Academy Medical Device Academy
Http Www Intertek Com Uploadedfiles Intertek Divisions Industrial Services Media Pdf Quick Guide For Major Medical Markets Pdf
Overview Of The Regulatory System For Medical Devices In Kenya
5 Tips For Medical Device Registration Across Global Markets
Health Canada Introduces New Reporting Requirements To Reduce Shortages 2021 04 14 Fdanews
Guidance On Medical Device Establishment Licensing Gui 0016 Canada Ca
Medical Device Regulations Classification Submissions
2018 2019 Report On Fees Canada Ca
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca




Posting Komentar untuk "Health Canada Medical Device Establishment License Database"