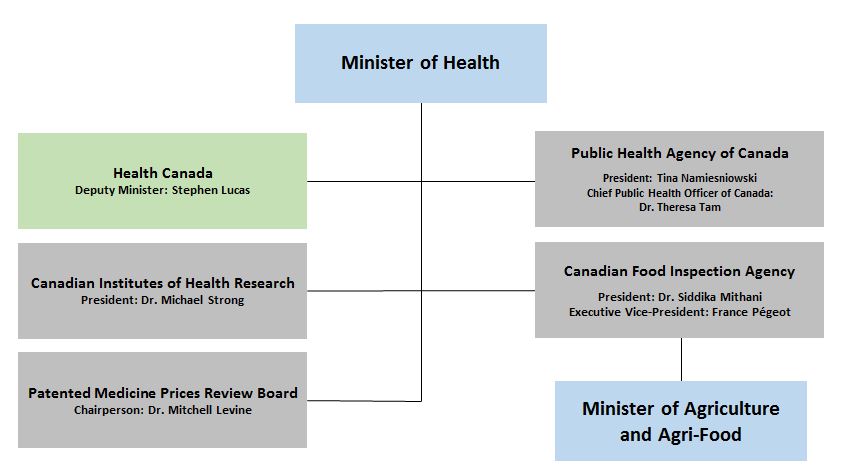
Mdsap Health Canada
Meander 1051 Arnhem 6825 MJ Netherlands. Medical Device Single Audit Program MDSAP audit timeframes have been reduced in response to industry feedback received by Health Canada.
Eu Mdr 2017 745 Technical Documentation Template In 2021 Technical Documentation Medical Device Medical
MDSAP includes compliance with the QMS requirements of the Canadian Medical Devices Regulations CMDR.

Mdsap health canada. The Pilot is scheduled to conclude December 31 2016 and as stated in Health Canada Notices dated January 2014 and January 16 2015 Health Canada intends to implement MDSAP as the sole mechanism for manufacturers to demonstrate compliance with the quality management system requirements of the Medical Devices Regulations the Regulations. UPDATE Health Canada had originally stated that participation in MDSAP would be mandatory from 1st January 2019 onwards. On May 9 2018 FDA participated in the MDSAP Stakeholder Day with the Therapeutic Goods Administration of Australia Brazils National Health.
List of Registrars Registrar Contact Information BSI Group America Inc. However due to practical difficulties involved with achieving this deadline Health Canada issued a notice on 13th April 2018 stating that manufacturers will be able to continue selling their product in Canada after 1st January 2019 provided they have an existing CMDCAS ISO 13485. Among other benefits this will support Canadian companies in expanding their business to other markets and reduce red tape for those wishing to operate in multiple markets.
Demonstrate Commitment to Quality MDSAP provides a comprehensive quality management system focused on areas directly impacting safety performance and reliability in the medical device lifecycle. Food and Drug Administration FDA. To facilitate uninterrupted market access TÜV SÜD will no longer be conducting CMDCAS audits as of September 30 2017.
Canada has made it mandatory to obtain an MDSAP certification by January 1st 2019 in order to continue to supply products to Canada. The MDSAP is an audit program that allows auditing organization to perform a single audit of a medical device manufacturer to obtain a certification that takes in consideration the applicable regulatory requirements of each of the partecipating countries. The transition to the MDSAP was initially announced in December 2015 and was completed in 2019.
Health Canada will terminate the CMDCAS program on December 31 2018. All manufacturers holding medical device licences in Canada now participate in the program which improves Health Canadas oversight of the medical devices sold in Canada and ensures that the medical devices Canadians use meet higher quality standards. The MDSAP process may nominally take 6 months but could take much longer so manufacturers who havent already done so must act now.
Therefore manufacturers wishing to place a product on the market in Canada need to have MDSAP Certification issued by an AO. All regulatory authorities participating in the MDSAP are equal partners in. MDSAP compliance is effective as of January 1 2019.
MDSAP which allows firms to undergo one audit by an accredited third party to satisfy quality regulations for Canada the US Brazil Japan and Australia officially replaced the traditional Canadian Medical Devices Conformity Assessment System CMDCAS audits on Jan. Medical Device Single Audit Program MDSAP The MDSAP is an audit program that is composed of the following five jurisdictions. 12950 Worldgate Drive Suite 800 Herndon VA 20170 USA.
Manufacturers transitioning to MDSAP during a surveillance audit need to demonstrate to Health Canada that. They have undergone an initial or recertification audit to ISO 13485 under CMDCAS on or after January 1 st 2016 They hold a valid ISO 13485 certificate issued under CMDCAS that has a validity period to at least December 31 st 2018. 16 rows List of Registrars Recognized by Health Canada under section 321 of the Medical Devices Regulations MDR Date of Issue.
USA Australia Canada Japan and Brazil. MDSAP audit time reductions will primarily target smaller medical device companies with limited regulatory compliance resources. Certification of your MDSAP-compliant quality system by a Health Canada and MDSAP-accredited Auditing Organization AO is also required before your device can be sold in Canada.
When certifying to the MDSAP you enable regulatory access to sell your device in Australia Brazil Canada Japan and the United States. Health Canada Health Canada HC will ONLY accept MDSAP for manufacturers who market their devices in Canada. After this date Health Canada will only accept MDSAP certificates from manufacturers with their device applications or renewals.
The Government of Canada is transitioning to the Medical Device Single Audit Program MDSAP. Health Canada uses MDSAP certificates as evidence of conformity to Medical Devices Regulations sections 32 2 f 32 3 j and 32 4 p. Health Canada had flagged this fact as early as December 2015 but it went somewhat under the radar until the RAPS meeting in September 2017 when representatives from Health Canada stated that the 1 st January 2019 deadline would not change.
Health Canada MHLWPMDA and the US.
Best Tips Iso 13485 Procedures With Our Free Template Version 2016 Iso 13485 Iso Procedure Template
Prrc Or Person Responsible For Regulatory Compliance Mdr 2017 745 Medical Humor Regulatory Compliance Regulatory Affairs
What Is A Medical Device Official Definition For Eu Usa China Brazil Medical Medical Device Medical Equipment
Podcast Episode 3 Is My Product A Medical Device In Europe
Pin On Medical Devices Regulation
Mett With Me At Topra Symposium We Can Discuss About Easy Medical Device Or Also If You Have Some Projects Where You Would Need My Support Looking Forward To
Mdsap Infographic Medical Device Medical Medical Supply Storage
11 Questions Sur Le Mandataire Europeen Dispositif Medicaux Medical Med Tech Medical Device
Six Elements Of Organizational Design Organizational Design Organizational Design
Pin On Medical Device Infographics
Green Belt Certificate 3rd Edition Regulatory Affairs Green Belt Regulatory Compliance
Mdsap Infographic Medical Device Medical Medical Supply Storage
Pin On Medical Devices Regulation
Pin On Association For Vascular Access
How To Place A Custom Made Medical Device On The Market Medical Medical Device Regulatory Affairs
How To Write A Declaration Of Conformity Mdr And Ivdr Declaration Regulatory Affairs Conformity
Semoegy Medical On Twitter Medical Device Medical Information Medical
Infusion Nurses Society Leader Discusses Improving Care With Ivwatch Ceo Nursing Associations Pediatric Nursing American Nurses Association



Posting Komentar untuk "Mdsap Health Canada"