Health Canada Interim Order List
This Interim Order allows Health Canada and the Canadian Food Inspection Agency to address critical supply issues in an expedited manner when shortages occur. In particular the document refers to the following interim orders.
Https Www Epa Gov Sites Production Files 2015 01 Documents Dfe Interim Fragrances Criteria Pdf
Healthcare professionals can request access to COVID-19 related medical devices not yet licensed in Canada or authorized for sale under the Interim Order pathway through Health Canadas Special Access Program SAP.
Health canada interim order list. By John Greiss and Kristin Wall on December 3 2020 Posted in Life sciences and healthcare Pharmaceuticals and life sciences. The Interim Order allows importers holding Canadian drug establishment licenses DELs to import designated products without meeting all of the requirements of the Canadian Food and Drug Regulations. Medical Face Mask 3-ply I.
Health Canada maintains and updates the list as required. Health Canada has published an Interim Order Respecting Drugs Medical Devices and Foods for a Special Dietary Purpose in Relation to COVID-19 the Interim Order. On March 30 2020 the Canadian Minister of Health issued the Interim Order under section 3011 of Canadas Food and Drugs Act.
Applications are considered on a case-by-case basis. List of applications received. In particular importers are exempt from meeting a.
Dec 2 2020. Its the same list that was incorporated by reference in the previous IO for the exceptional importation and sale of drugs. 11 rows Cardinal Health.
This order allows Health Canada and the Canadian Food Inspection Agency to address critical supply issues in an expedited manner when shortages occur. Health Canada has published an Interim Order Respecting Drugs Medical Devices and Foods for a Special Dietary Purpose in Relation to COVID-19 the Interim Order. This order allows Health Canada and the Canadian Food Inspection Agency to address critical supply issues in an expedited manner when shortages occur.
Health Canada consults on transition of interim order drugs vaccines and medical devices to permanent approval pathways. As indicated in a previous blog post Health Canada HC announced its intention to leave several Interim Orders relating to COVID-19 in place until at least the fall of 2021. Health Canada has published a notice summarizing the most important aspects related to the Interim Orders IOs in the context of the outbreak of COVID-19 and special regulatory measures associated thereto.
23 rows Drug and vaccine authorizations for COVID-19. By this time the agency plans to have regulatory amendments in place that will maintain a balance between flexibility and regulatory oversight. Health Canada has published an Interim Order Respecting Drugs Medical Devices and Foods for a Special Dietary Purpose in Relation to COVID-19 the Interim Order.
As we have previously reported the Minister of Health Minister approved the Interim Order Respecting the Importation Sale and Advertising of Drugs for Use in Relation to COVID-19 Interim Order. Information on the SAP for medical devices can be found here. Non Sterile Face Mask.
Health Canada has published an Interim Order Respecting Drugs Medical Devices and Foods for a Special Dietary Purpose in Relation to COVID-19 the Interim Order. Cross Protection M SDN. Drugs included on this list are called designated drugs and are eligible for the exceptional importation and sale provisions provided for in Interim Order No.
Surgical Face Mask with ear loops. 120 rows The Interim Order Respecting Drug Shortages introduces new measures to help safeguard. The Interim Order offers an opportunity for manufacturers to answer Canadas pressing call for required medical supplies and to test the waters of the Canadian market while shortages last.
Interim order importation and sale of medical devices. This Interim Order allows Health Canada and the Canadian Food Inspection Agency to address critical supply issues in an expedited manner when shortages occur. To date Health Canada has granted authorizations under the Interim Order for the Pfizer-BioNTech COVID-19 Vaccine December 9 2020 the Moderna COVID-19 Vaccine December 23 2020 the AstraZeneca COVID-19 Vaccine February 26 2021 and the Janssen COVID-19 Vaccine March 5 2021 among others.
According to the official notice starting from March 2020 Health Canada has already issued 5 IOs dedicated to the most important matters in the context of the COVID-19 outbreak. The document is intended to provide medical device manufacturers and other parties involved with additional information and clarifications regarding. On March 30 2020 the Canadian Minister of Health issued the Interim Order under section 301 1 of Canadas Food and Drugs Act.
Taizhou Rich Medical Products Co Ltd.
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca
Https Apps Who Int Iris Bitstream Handle 10665 259481 9789241210157 Eng Pdf
Https Www Un Org Esa Coordination Cl 14 Final For Printing Pdf
Https Www Who Int Docs Default Source Medicines Regulatory Updates Covid 19 28th Who Regulatory Update On Covid 19 05feb2021 With Revision Pdf Sfvrsn 7f51c8a8 5 Download True
Temporary Fixes To Chronic Drug Shortages Leave Canada Vulnerable Cmaj News
Questions And Answers Access To Drugs In Exceptional Circumstances Canada Ca
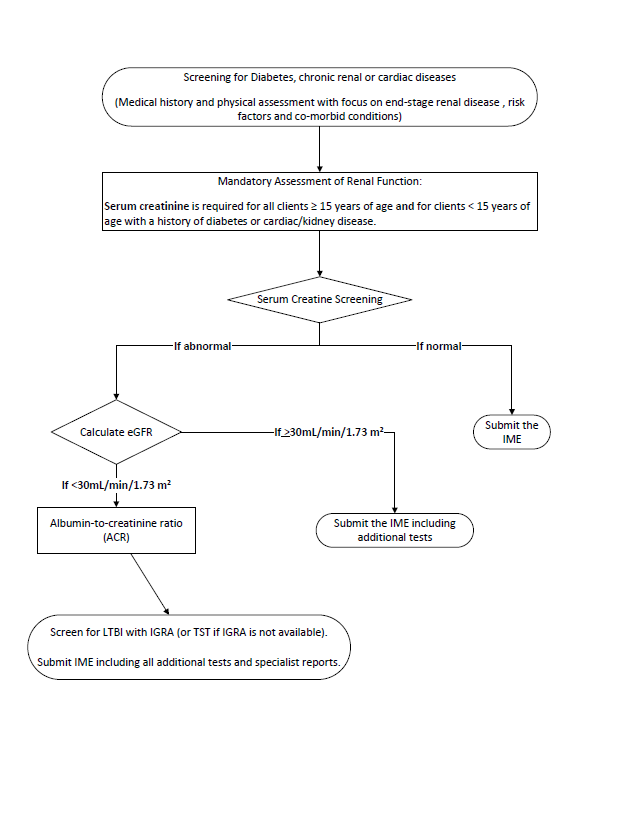
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Https Www Who Int Diagnostics Laboratory Eual 200110 New Eul Procedure Final Pdf Ua 1
Https Www Astrazeneca Ca Content Dam Az Ca Downloads Productinformation Az Covid 19 Hprc Us Labelled Supply 2021 03 31 En Pdf
Interim Guidance On The Use Of Abbott Panbio Covid 19 Antigen Rapid Test Ccdr 47 1 Canada Ca
Canadian Panel Member Guide To Immigration Medical Examinations 2020 Canada Ca
Applications For Medical Device Investigational Testing Authorizations Guidance Document Canada Ca
Authorization Of Covishield With English Only Vial And Carton Labels Recalls And Safety Alerts
From Regulatory Flexibility To Reimbursement Changes How Canadian Regulators And Payers Are Managing The Covid 19 Crisis
Resettlement Assistance Program Rap Service Provider Handbook Canada Ca
Https Www Nortonrosefulbright Com Media Files Nrf Nrfweb Knowledge Pdfs Essential Services And Essential Workers In Canada Pdf
Https Www Astrazeneca Ca Content Dam Az Ca Downloads Productinformation Az Covid 19 Hprc Us Labelled Supply 2021 03 31 En Pdf




Posting Komentar untuk "Health Canada Interim Order List"