Health Canada Botox Regulations
Botox Cosmetic injections should only be given by a qualified health care provider no more frequently than every two months according to the Canadian product monograph CPM. An issue has arisen in Canada over the unlicensed administration of Botox by health spas.
Latisse Skintology Health And Wellness Centre
As an RN can I administer botulinum toxin eg.
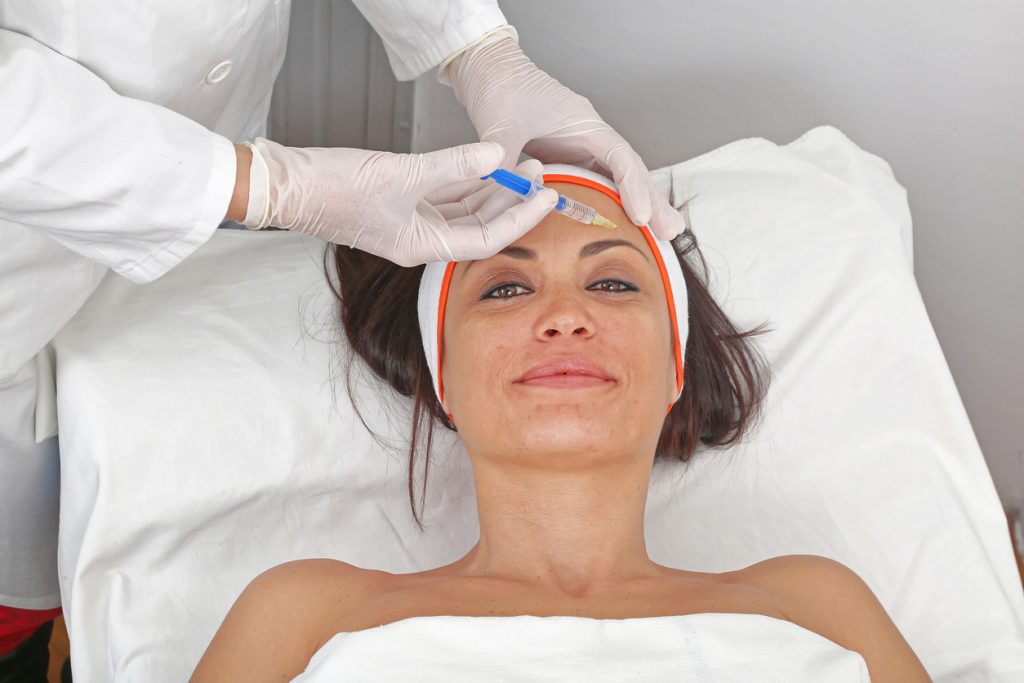
Health canada botox regulations. The issue involves health spas in the Vancouver area who are ignoring Health Canada regulations stating that Botox should only be prescribed and injected by a medically qualified physician. Consumers and health professionals are also encouraged to report adverse reactions. Physicians 92 of physicians performing botulinum toxin have no formal training in the field of botulinum toxin nor guidelines laid out by their regulatory body.
If you are using Botox Cosmetic products you should seek immediate medical care if. Dispose of any unused saline. Botox must be refrigerated at a temperature between 2 and 8ºC in its diluted form and must me used within a period of 24 hours.
The veterinary labelling is developed by the drug sponsor according to the Food and Drug Regulations. The prescriber also has responsibility for the outcome of the procedure. When receiving Botox treatments ask your health care professional to show you the product label and verify that the health product has been authorized for sale by Health Canada.
Dont risk your health with DIY Botox. Health Canada has approved BOTOX onabotulinumtoxinA manufactured by Allergan Inc. This is one of the controlled acts that RNs and RPNs are allowed to perform with an order from an authorized health care professional such as a physician.
Administering botulinum toxin falls under the controlled act of administering a substance by injection. The manufacturer and importer must. They must be present at a patient consultation.
If you have Botox injections again you should wait at least 3 months. Only medical professionals can qualify as prescribers so therapists can only perform Botox injections if they work alongside a prescribing clinician. One of our readers kindly sent in the following comment to add to this article.
The recommended dilution is 100 Units10 mL with preservative-free 09 Sodium Chloride Injection USP see Table 1. As a prophylactic preventive treatment for headaches in adult. Dentists are generally required to take post-graduate training in order to provide Botox and dermal fillers.
The prescriber cannot dispense the toxin remotely. Yes you may administer botulinum toxin if there is an appropriate order in place. Some Vancouver-area medical spas are ignoring Health Canada regulations that Botox be prescribed and injected by a physician a CBC News investigation.
Depending on your location different state laws and regulations are surrounding various procedures. Before dilution vials of Botox Cosmetic are stored in a freezer at a. Physicians are not required to take any additional training in order to provide botulinum toxin or dermal fillers.
The veterinary labelling is developed by the drug sponsor according to the Food and Drug Regulations. Manufacturers are required to provide Health Canada with reports of serious adverse reactions for health products they sell in Canada. Provide a list of the products ingredients notify Health Canada that.
The recommended dose is 100 Units of Botox and is the maximum recommended dose. The product monograph is developed by a drug sponsor according to guidelines published by Health Canada that provide direction on the content and format. Your practitioner should be able to give you more advice about what you should and should not do.
FYI Clients who choose to receive BOTOX or DYSPORT injections must see an authorized doctor annually for a check up to ensure they are a candidate for. While Health Canada reviews the product monograph or the veterinary labelling as part of the drug review process it remains the responsibility of the drug sponsor to ensure that the product monograph or the veterinary labelling is complete and accurate. Also avoid vigorous exercise sunbathing including using sunbeds and the sauna for 2 days.
Treatment failure is defined as using optimal doses of the indicated number of anticholinergic drug s for NDO or anticholinergic drugs andor mirabegron for OAB for a minimum of 4 weeks on each drug without a reduction of symptoms. The Cosmetic Regulations and the Food and Drugs Act require that cosmetics sold in Canada are manufactured prepared preserved packed and stored under sanitary conditions. While Health Canada reviews the product monograph or the veterinary labelling as part of.
The effects usually last for about 3 or 4 months. In Canada Botox treatments should only be provided or recommended by licensed medical physicians dentists or registered nurses who have also obtained relevant training in safely and effectively administering Botox and dermal fillers.
Coronavirus No Botox And No Fillers Under Lockdown Bbc News
Qualifications Needed To Open A Med Spa Apt Injection Training
Lip Fillers Perfect Storm Due To Lack Of Regulation Bbc News
Botox Training Courses For Dentists Ptifa
Coronavirus No Botox And No Fillers Under Lockdown Bbc News
Who Can Administer Dermal Fillers In Canada Dr Dargie Medical Aesthetics
Aesthetics Market Index Your Practice Allergan
Botox In Your Dental Office Botox Education Training News
Https Www Plasticsurgery Org Documents Health Policy Positions Injectables And Fillers Legal And Regulatory Risk Pdf
Dentists Advertising Botox Injections Urged To Be Legally Compliant Dental Tribune Uk Ireland
Administering Botulinum Toxin Injections Who Is Qualified Iapam
Botulinum Toxin And Cosmetic Fillers Children Bill House Of Lords Library
Wrinkle Relaxing Injections Vancouver Laser Skin Care Centre
Allergan Botox Training Course Hands On Cme Certified Iapam
Botox Approved To Treat Migraines In Canada The Globe And Mail




Posting Komentar untuk "Health Canada Botox Regulations"