Health Canada Medical Device Recall Database
Recalls mandatory problem reporting handling storage delivery installation and servicing. Report a side effect.
Https Academic Oup Com Jtm Article Pdf 25 1 Tax092 25833991 Tax092 Pdf
A correction or removal action taken by a manufacturer to address a problem with a medical device.

Health canada medical device recall database. Report a serious adverse drug reaction for hospitals Report a medical device problem for health care professionals Prescription Drug List. Recalls occur when a medical device is defective when it could be a risk to health or when it is both defective and a risk to health. You can also check whether products have been authorized for sale by searching the Drug Product Database Medical Devices Active Licence Listing MDALL and Licensed Natural Health Product Database.
Reference Canadas Medical Device Regulations SOR-98-282 and Guidance Document for Mandatory Problem Reporting for Medical Devices for the correct reporting timeline which will depend on the severity of the incident. Health Product InfoWatch Canada Vigilance adverse. HOME-BOND and VIA-BOND Epoxy products recalled.
Medical device incident reporting investigation scheme IRIS Database of Adverse Event Notifications DAEN Medical devices safety Medical Devices Safety Update. Report complaints involving medical devices to Health Canada by calling toll-free at 1-800-267-9675 or by reporting online. About the Drug and Health Product Register.
Canadian data is current through March 2018. Submit a Preliminary Report to Health Canada detailing the incident and corrective action strategy. Advisories Warnings and Recalls - MedEffect Canada.
Data and review decisions. Only products which appear in this database listing may be offered for general marketing purposes in Canada. Recent recalls and alerts.
Unauthorized drugs seized from Tokyo Beauty in Burnaby BC may pose serious health risks. The Recalls and Safety Alerts Database provides easy access to a comprehensive list of recalls advisories and safety alerts. Recalls and safety alerts.
Because the RSAD is incomplete for medical devices due to the time required to backfill records we also obtained complete information on all medical device recalls. Report any health product-related adverse reactions or complaints to Health Canada. Per the guidance document Distributor a person other than a manufacturer an importer or a retailer who sells a medical device in Canada for the purpose of resale or use other than for personal use.
Contact your healthcare provider if you have any health concerns related to your use of a medical device. Contact Health Canada at 1-800-267-9675 or by completing an online complaint form if you find a drug product that contains gentian violet in the Canadian marketplace. All lots of Yummy Sports Candies BCAA powder all flavours recalled due to missing safety information for pregnant and breastfeeding women.
This database includes recalls from Health Canada the Canadian Food Inspection Agency and Transport Canada. 2021-07-06 Health products COVID19. Martin Clinic Liquid Vitamin D recalled due to incorrect dosing and missing safety information on the label.
A correction or removal action taken by a manufacturer to address a problem with a medical device. Recent health products recalls and alerts. This database includes recalls from Health Canada the Canadian Food Inspection Agency and Transport Canada.
An MDEL provides Health Canada assurance that medical devices sold or imported into Canada meet the safety requirements set out. Recalls and safety alerts are sent out when we have important information to sharemeaning you can feel more secure when choosing and using products. The Bureau maintains a database of all licensed Class II III and IV medical devices offered for sale in Canada.
Nestlé brand Drumstick Vanilla Chocolate Swirl Non-Dairy Frozen Dessert Cones recalled due to undeclared milk. Health Canada Medical Device. The Recalls and Safety Alerts Database provides easy access to a comprehensive list of recalls advisories and safety alerts.
Recalls occur when a medical device is defective when it could be a risk to health or when it is both defective and a risk to health. Use the Medical Device Recall Reporting Form - Initial FRM-0360 to complete your initial recall report. Class I medical devices do not require a medical device licence and are monitored by the Health Products and Food Branch Inspectorate Compliance and Enforcement through Establishment Licensing.
When a product is recalled or an advisory or alert is issued it means our surveillance tools are working. Use the Medical Device Recall Reporting Form - Final FRM-0360 to complete your final recall report. Stay connected with Health Canada and receive the latest advisories and product recalls.
2021-07-07 Health Products. Canadian data is current through March 2018. A Medical Device Establishment Licence MDEL is a licence issued to Class I manufacturers as well as importers or distributors of all device classes to permit them to import or distribute a medical device in Canada.
Submit a section 65 final report to your nearest Health Canada regional office as soon as possible after completing the recall. We collected information on medical device recalls for the 10-year period January 1 2005 to December 31 2014 using the Recall and Safety Alerts Database RSAD which contains information on recalls and safety alerts related to consumer products vehicles food and health products in Canada.

Pdf How Are Allied Health Notes Used For Inpatient Care And Clinical Decision Making A Qualitative Exploration Of The Views Of Doctors Nurses And Allied Health Professionals

Incident Reporting For Medical Devices Guidance Document Canada Ca

Nurse Practitioner Resume Example Nursing Resume Template Nursing Resume Examples Nursing Resume
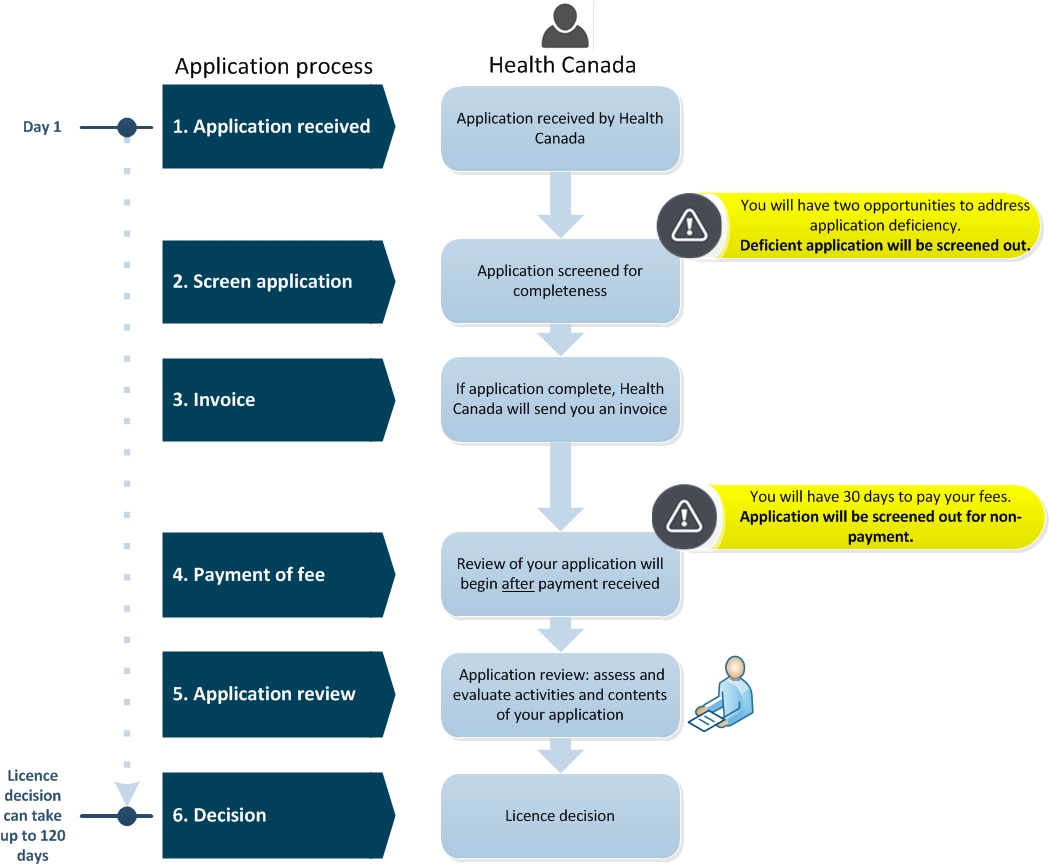
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca

Drug And Medical Device Highlights 2018 Helping You Maintain And Improve Your Health Canada Ca

125 Good Hospital Slogans And Taglines Slogan For Health Medical Quotes Health Slogans

Israel Medical Device Regulations Regdesk

Guidance On Medical Device Establishment Licensing Gui 0016 Canada Ca

Cbc News S Joint Investigation Into The Sometimes Murky World Of Medical Devices Cbc News

Ctrl An Online Dynamic Consent And Participant Engagement Platform Working Towards Solving The Complexities Of Consent In Genomic Research European Journal Of Human Genetics

Mddi Medical Device And Diagnostic Industry News Products And Suppliers Digital Health Medical Device Medical

Research Assistant Resume Example Medical Assistant Resume Education Resume Resume Examples
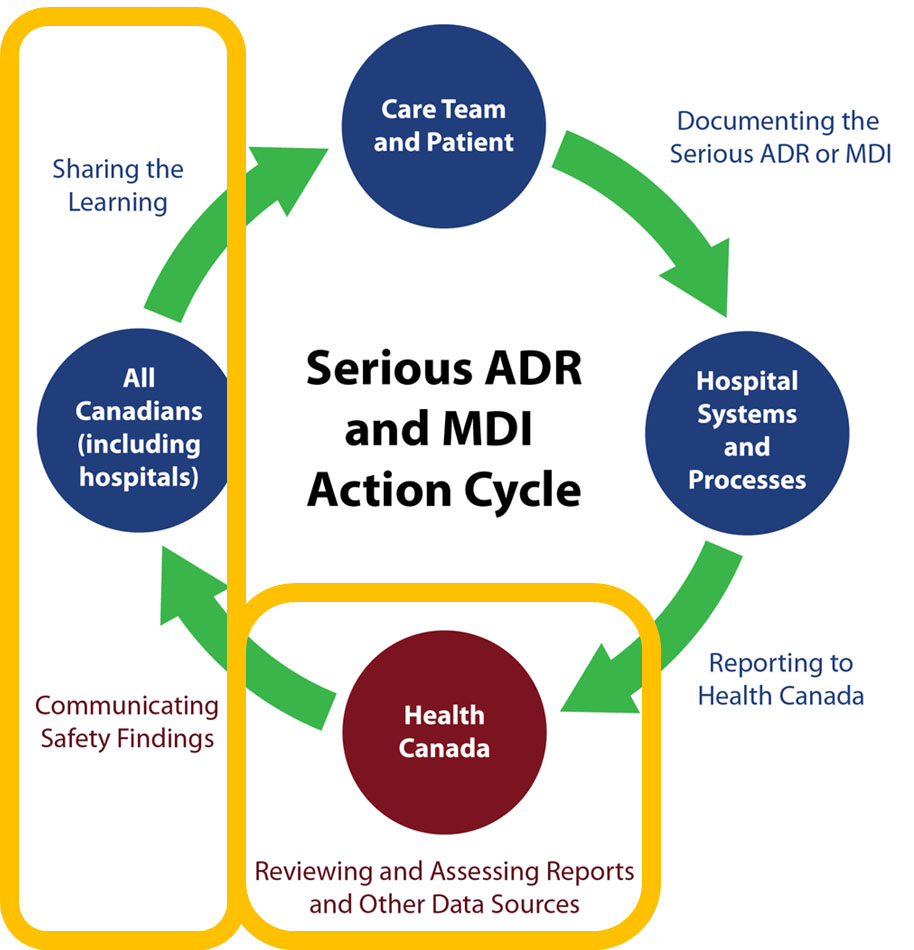
Module 4 Health Canada S Review And Communication Of Safety Findings Canada Ca

List Of Recognized Standards For Medical Devices Canada Ca
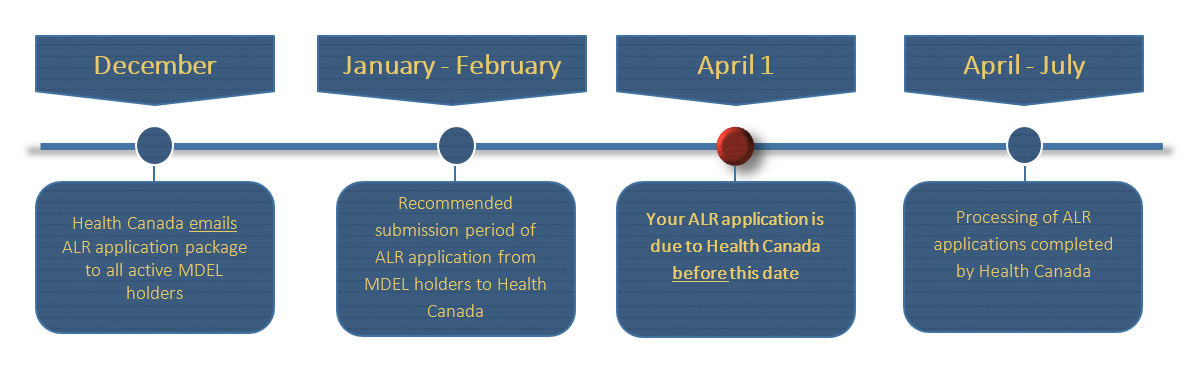
Frequently Asked Questions Medical Device Establishment Licensing And Fees Canada Ca

Drug And Natural Health Products Recall Guide Canada Ca

.png.aspx)
Posting Komentar untuk "Health Canada Medical Device Recall Database"