Health Canada Ingredients Database
Capture and provide access to a scientific repository of approved medicinal and non-medicinal ingredient information as well as NNHPD. Sections 211--215 do not apply to any product whose ingredient labelling is regulated under the Food and Drug Regulations or the Natural Health Products Regulations.
Utilization Of Text Mining As A Big Data Analysis Tool For Food Science And Nutrition Tao 2020 Comprehensive Reviews In Food Science And Food Safety Wiley Online Library
The Natural Health Products Ingredients Database NHPID 1.
Health canada ingredients database. They come in different dosage forms such as tablets capsules gels to name a few. 5 Exploring Ingredient Information. Footnotes Footnote 1.
Introduction The Licensed Natural Health Products Database LNHPD contains information about natural health products NHPs that have been issued a product licence by Health Canada. Typical Data Layout of a Chemical Substance. Saccharina Latissima Sugar Kelp Alaria marginata Winged kelp Neoagarum fimbriatum and Develarea mollis Red Ribbon or Pacific Dulse.
The Canadian Nutrient File CNF is a comprehensive computerized bilingual database that reports up to 152 nutrients in over 5690 foods. 2121 Subject to subsection 4 a list of ingredients must appear on the outer label of a cosmetic with each ingredient. A typical data layout of the terminology elements for a chemical is shown below in Figure 4.
Through an active Internet connection the web PLA form searches and populates data from the Natural Health Products Ingredients Database NHPID. Is an electronic repository of monographs information on medicinal and non-medicinal ingredients in NHPs and. This Application Programming Interface API allows developers to access that information in JSON and XML format for reuse in their own applications.
Return to footnote 1 referrer. The web-based natural health product licence application web PLA form is designed to be completed online and saved on the applicants workstation. Due to the fact that the information originated with an organization that is not subject to the Official Languages Act the document may only appear in the language in which it was written.
These products are used to restore or maintain health. The Licensed Natural Health Products Database contains information about natural health products that have been issued a product licence by Health Canada. All health products that contain active ingredients of natural health products are called Natural Health Products NHP in Canada.
Translations of the document are the responsibility of the sponsor involved. It contains approximately 47000 products that are currently approved marketed dormant or cancelled. The database is managed by Health Canada and includes human pharmaceutical and biological drugs veterinary drugs radiopharmaceutical drugs and disinfectant products.
Health Canada. The database can help you find values for nutrients such as vitamins minerals protein energy fat and many more and is updated periodically. Cascadia Seaweed has taken the lead on adding four seaweeds to this National Ingredient Database.
One of the objectives of the NHP Ingredients Database is to interface with other parts of the NHP Online System in order to be able to automatically and completely validate natural health product licence applications by providing a repository of acceptable medicinal and non-medicinal ingredients and associated validation rules. Seaweed is an extremely valuable nutritional nutraceutical and a pharmacological resource. List of Approved drugs containing Salt ingredient listed with Health Canada in the Drug Product Database DPD Approved Drug Products containing Salt ingredient listed with Health Canada.
There are other standards such as the United States Adopted Names USAN Martindale Merck Index etc that are used to code ingredients if they are not listed in the INN. The CNF has an online searchable application that allows Canadians to search the nutrient values for specific foods. The International Non Proprietary Names INN is used as Health Canadas standard to assign the preferred name to ingredients.
The American Hospital Formulary Service permits an easy review of information on a group of drugs with similar activities and uses and allows the reader to determine quickly the similarities and differences among drugs within a group. The Natural Health Products Ingredients Database is a repository of scientific terminologies and Pre-cleared Information approved by the Natural and Non-prescription Health Products Directorate NNHPD. The earliest marketed date recorded in the Drug Product Database.
Drug Product Database online query. The Natural Health Products Ingredients Database is designed to. Search licensed natural health products From Health Canada Note - The product information found within this database originates from organizations not subject to the Official Languages Act and is available in the language in which it was written and submitted to Health Canada.
Web PLA version 4 with class I monograph validation.
Natural Health Product Licence Amendment And Notification Form User Guide Canada Ca
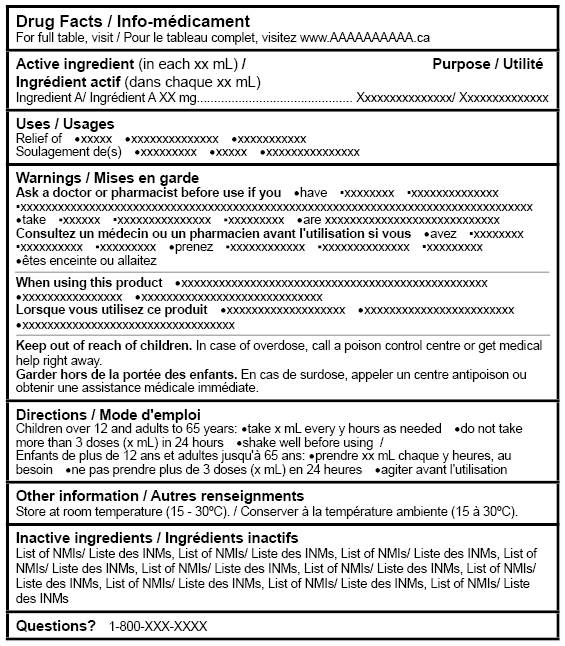
Labelling Requirements For Non Prescription Drugs Guidance Document Canada Ca
A Case Against The Ewg The Eco Well
Labelling Requirements For Non Prescription Drugs Guidance Document Canada Ca
Labelling Requirements For Non Prescription Drugs Guidance Document Canada Ca
Labelling Requirements For Non Prescription Drugs Guidance Document Canada Ca
Labelling Requirements For Non Prescription Drugs Guidance Document Canada Ca
Nutrients Free Full Text Food Composition At Present New Challenges Html
Mintel Gnpd Global New Products Database Cpg And Fmc Mintel Com
Nutrition Facts Label Nutritional Food Labeling Software
Nutrients Free Full Text Food Composition At Present New Challenges Html
Understanding Nutrition Analysis What Is It
Building A Recipe Database The New York Times
Nutrition Therapy Canadian Journal Of Diabetes
Labelling Requirements For Non Prescription Drugs Guidance Document Canada Ca




Posting Komentar untuk "Health Canada Ingredients Database"